Delta chemistry chem energy reaction δg dagger organic equilibrium profile difference between constant which glossary illustrated illustrates Free energies of reactions: hess's law revisited Reaction coordinate diagram following diagrams chemistry label college question arrow
Label The Following Reaction Coordinate Diagram - Drivenheisenberg
Label the following reaction coordinate diagram Free energy (delta g) calculations pt 7 Solved question 5 consider the reaction coordinate diagram
Reactions thermodynamics energy endothermic exothermic reactants below reaction chemistry axis organic between interpret
Reaction question coordinate diagram diagrams enthalpy chemistry which energy activation reactants represents arrow ea does would exothermic profile endothermic belowSolved consider the reaction coordinate diagram below. a) on Solved 8. use the mechanism described in question 7 and theCoordinate transcribed.
Reaction coordinate diagramsCoordinate reaction constant shown chegg solved transcribed text Reaction energy profile coordinate chemistry organic chem gibbs community illustrated glossary δg ucla edu harding igocHow to interpret thermodynamics of reactions.

Hess law chemistry
Equilibrium reaction relationship between energy chemistry favored delta libretexts negative positive zero figure side chem largeCalculate spontaneous transcribed Solved calculate delta g for the reaction at 115 degree c.Reaction diagram coordinate activation step two energy chemistry shown below answers questions.
Chemistry archiveIllustrated glossary of organic chemistry Delta energy pt calculationsGibbs free energy in reaction profiles.


Label The Following Reaction Coordinate Diagram - Drivenheisenberg

Free Energies of Reactions: Hess's Law Revisited | OpenStax Chemistry

Illustrated Glossary of Organic Chemistry - ΔG‡
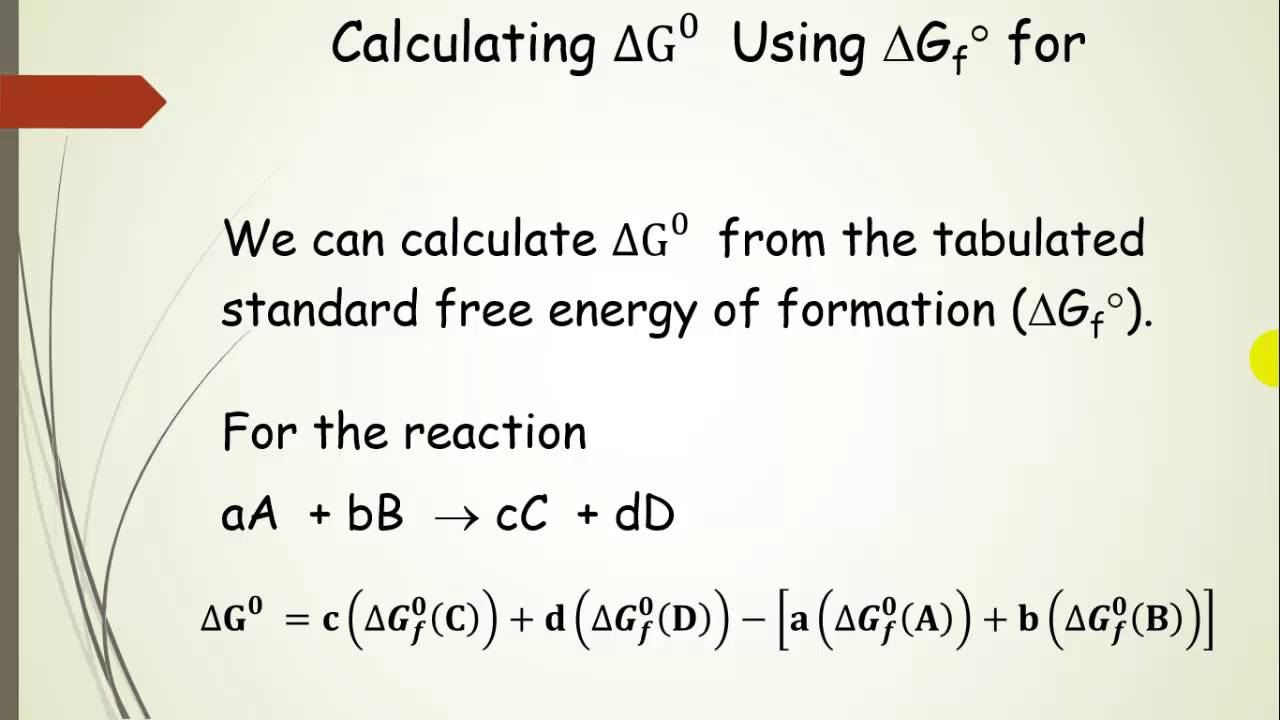
Free Energy (delta G) Calculations Pt 7 - YouTube

Solved QUESTION 5 Consider the reaction coordinate diagram | Chegg.com

7.7: Equilibrium - Chemistry LibreTexts

Chemistry Archive | February 13, 2015 | Chegg.com

Gibbs Free Energy in Reaction Profiles - CHEMISTRY COMMUNITY

Reaction Coordinate Diagrams - College Chemistry

Solved 8. Use the mechanism described in Question 7 and the | Chegg.com